We use cookies to ensure that we give you the best experience on our website. If you continue to use this site we will assume that you are happy with it.
Why Positive Pressure Cascades are Essential in GMP Pharmaceutical Cleanrooms
BY: Royce Tourtillott

In pharmaceutical manufacturing, contamination control is a non-negotiable priority. Cleanroom environments must be designed to meet the strict standards of Good Manufacturing Practice (GMP)—protecting not only the products but the patients who rely on them. One of the most effective tools in achieving this level of control is the implementation of a positive pressure cascade strategy.
At Life Sciences Solutions, we design cleanroom systems that leverage proven airflow strategies—like positive pressure cascades—to maintain compliant, contamination-free environments. Here’s what this strategy involves and why it’s vital for GMP-regulated pharmaceutical facilities.
What Is a Positive Pressure Cascade?
A positive pressure cascade is a cleanroom airflow strategy that maintains higher air pressure in cleaner, more critical areas and progressively lower pressure in less critical adjacent spaces. The goal is to create a directional flow of clean, filtered air outward—from the most critical zones (often the aseptic core) to less critical environments.
Example of pressure differential hierarchy:
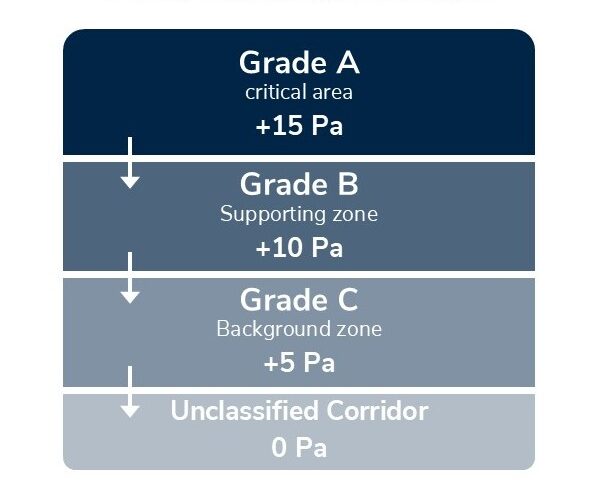
This creates a pressure “cascade” that prevents contaminated air from entering high-grade spaces. When a door opens, air naturally flows outward—pushing contaminants away, rather than drawing them in.
3 Reasons Positive Pressure Cascade Is Crucial in Cleanroom Design
Contamination in a cleanroom can originate from many sources—personnel, materials, or the surrounding environment. Airborne particles and microbes are especially difficult to control without a pressure strategy and the controlled airflow it creates.
Positive pressure helps to:
- Maintain Sterility in Aseptic Areas
Positive pressure ensures aseptic environments like fill-finish suites and Grade A zones remain uncompromised during operations and material/personnel transfers.
- Prevent Contamination Ingress
By maintaining higher pressure in critical areas, air flows outward into less clean zones—keeping dust, microbes, and particles from entering sterile spaces.
- Support GMP Regulatory Compliance
EU GMP Annex 1 and FDA 21 CFR Part 210/211 specifically emphasize pressure differentials as part of contamination control. Proper cascade design demonstrates adherence to these guidelines.
This airflow strategy is especially critical in aseptic processing areas such as fill-finish biologics and pharmaceutical manufacturing environments.
How It’s Implemented
Implementing a positive pressure cascade system requires careful planning and integration of advanced cleanroom HVAC technologies. Our engineering teams at LSS ensure every cleanroom is designed with:
- HEPA filtration systems to ensure an adequate supply of clean air
- Precision-controlled HVAC systems to supply a precise amount of air to achieve the desired room pressurization
- Airlocks and pass-through chambers to control airflow and allow for personnel and material movement within the cleanroom
- Pressure monitoring and alarm systems to monitor and detect deviations in real time
These systems work together to create a stable, compliant environment that protects product quality at every stage of manufacturing.
GMP Compliance: Meeting Regulatory Expectations
GMP guidelines, such as EU GMP Annex 1 and FDA CFR 21 Part 210/211, require effective contamination control through environmental management. Pressure differentials are often reviewed during audits and inspections, with non-compliance leading to major findings.
A properly designed and maintained positive pressure cascade system:
- Minimizes contamination risk
- Demonstrates GMP compliance
- Supports aseptic processing standards
- Reduces audit findings and operational risks
Our cleanroom designs not only meet regulatory expectations—they’re built to exceed them.
A Core Strategy for Pharmaceutical Manufacturing
The positive pressure cascade is not just a design detail—it’s a critical GMP strategy that safeguards cleanroom integrity. Whether you’re producing injectable drugs, high-purity APIs, or sterile packaging, your cleanroom must be equipped to defend against contamination.
At Life Sciences Solutions, we specialize in GMP-compliant cleanroom design and engineering. From airflow modeling to turnkey HVAC integration, our team ensures your facility is prepared for regulatory scrutiny and built for performance.
Ready to evaluate your pressure cascade strategy or upgrade your cleanroom?
Contact LSS to speak with a cleanroom expert.
